In scientific experiments, water is not only a solvent but also a critical factor determining the accuracy and reliability of results. However, not all types of water are suitable for every application. This article provides an overview of different types of laboratory water and guidance on selecting the most appropriate type for specific needs.
1. WHAT IS PURIFIED WATER?
Purified water refers to water produced by weak anion exchange resins, reverse osmosis (RO), or single distillation. Among these, reverse osmosis is the most commonly used method due to its cost-effectiveness and high efficiency. The electrical conductivity of purified water typically ranges from 1–50 µS/cm. However, to achieve higher purity levels, such as ultrapure water, electrolytes must be removed using techniques such as mixed-bed ion exchange and Electrodeionization (EDI). Purified water is commonly used for rinsing laboratory glassware, autoclaves, climate chambers, and laboratory washers.
2. HOW IS LABORATORY WATER PURITY EVALUATED?
To establish a unified classification system for water purity, several parameters are assessed, including electrical conductivity, resistivity, total organic carbon (TOC) content, bacterial contamination, endotoxin levels, and the presence of suspended particles. These criteria ensure that water meets the specific requirements of different research and analytical applications.
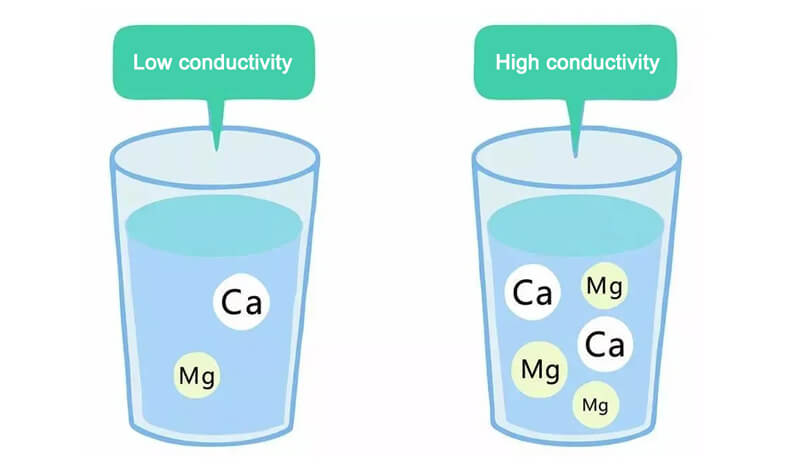
ELECTRICAL CONDUCTIVITY AND RESISTIVITY
Electrical conductivity is measured in microsiemens per centimeter (µS/cm) at 25°C and reflects the ion content of the water. Higher conductivity indicates higher ionic contamination, while pure or ultrapure water exhibits very low conductivity.
Resistivity, measured in megaohm-centimeter (MΩ·cm) at 25°C, is inversely related to conductivity. Higher resistivity indicates higher purity and lower ionic content. While conductivity is used to evaluate raw or drinking water, resistivity is primarily used for assessing ultrapure water.
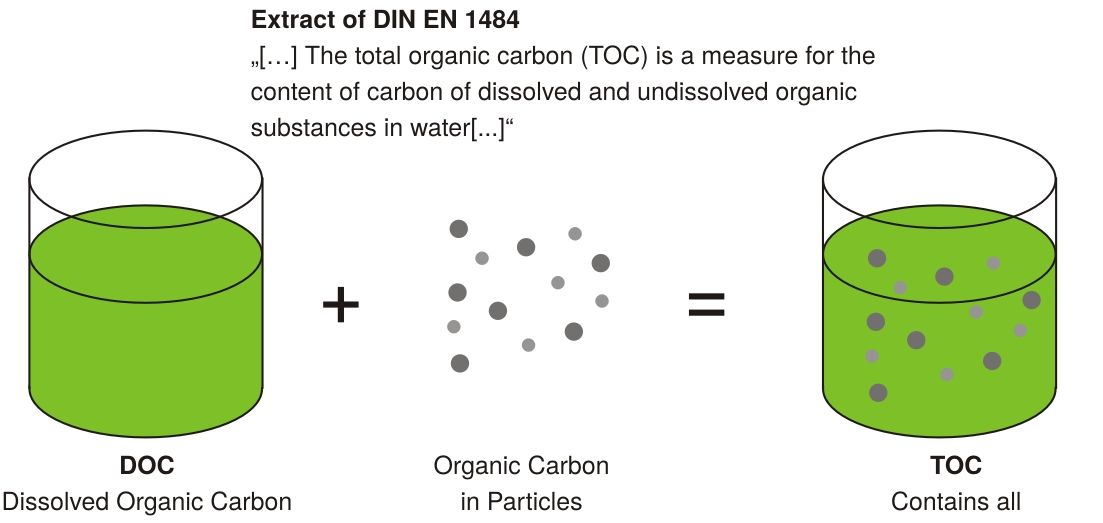
ORGANIC COMPOUND CONTENT
Water can contain various organic compounds. Measuring each individually is impractical; hence, Total Organic Carbon (TOC) is used to assess organic contamination. TOC quantifies the oxidized carbon resulting from organic compounds and is the primary indicator of organic impurity in laboratory water.
While chromatography can identify specific organic compounds, it is expensive and time-consuming, and thus not typically used for routine monitoring.
BIOLOGICAL CONTAMINATION
Bacteria and other microorganisms may be present in untreated water, leading to biological contamination. Bacterial levels are measured in colony-forming units per milliliter (CFU/mL). To minimize bacterial contamination, filtration, UV treatment, and chemical sanitization are employed.
Culturing techniques determine the overall bacterial load and specific types, while fluorescence-based methods can quickly differentiate between live and dead bacteria.
Besides bacteria, water can also contain endotoxins—substances released from Gram-negative bacterial cell walls. Endotoxin levels are measured in Endotoxin Units per milliliter (EU/mL), where 1 EU/mL is approximately equivalent to 0.1 ng/mL. Detection relies on the Limulus Amebocyte Lysate (LAL) assay.
PRESENCE OF COLLOIDS AND SUSPENDED PARTICLES
nsoluble particles cause turbidity, measured in Nephelometric Turbidity Units (NTU). Laboratory water must be highly transparent; thus, suspended particles are minimized using filtration systems.
Colloidal particles, smaller than 0.5 µm, may include iron, silica, aluminum, or organic matter. The Fouling Index (FI) evaluates the water’s potential to clog a 0.45 µm membrane filter.
3. CLASSIFICATION OF PURIFIED WATER
In laboratories, several grades of purified water are used, ranging from Type III water for equipment rinsing to Type I water for sensitive applications such as graphite furnace atomic absorption spectroscopy (GF-AAS). The correct water type directly impacts experimental accuracy and equipment performance. According to ISO 3696:1987, laboratory water is classified into three types:

TYPE I WATER (ULTRAPURE WATER)
Type I or ultrapure water approaches the theoretical purity limit. It is characterized by extremely low levels of organic matter, particulates, and microorganisms.
Produced through pre-treatment processes such as ion exchange, reverse osmosis, or distillation, followed by polishing with nuclear-grade ion exchange resins, Type I water features:
- Resistivity up to 18.2 MΩ·cm at 25°C
- TOC content <10 ppb
- Filtration down to 0.1 µm or smaller
- Bacterial levels <1 CFU/mL
Applications include high-precision analyses like High-Performance Liquid Chromatography (HPLC), Ion Chromatography (IC), and Inductively Coupled Plasma Mass Spectrometry (ICP-MS).
Due to its low endotoxin content, Type I water is also ideal for biological applications, including eukaryotic cell culture, typically employing ultrafiltration to remove pyrogens and enzymes.
TYPE II WATER
Type II water, also known as deionized water, may still contain non-ionic contaminants such as ethanol and pyrogens and a relatively higher bacterial load.
While unsuitable for injection preparations, it is widely used for glassware rinsing, reagent preparation, buffer dilution, and general laboratory use.
Type II water typically has a conductivity between 0.1 and 1.0 µS/cm, depending on the dissolved ionic content.
TYPE III WATER
Type III water is commonly produced by double-stage reverse osmosis systems. It features:
- Electrical conductivity ≤5.0 µS/cm
- pH between 5.0 and 7.0
Type III water is suitable for general laboratory applications such as glassware rinsing, autoclaves, climate chambers, and equipment washing.
| Specification | Grade 1 | Grade 2 | Grade 3 |
| Electrical Conductivity (µS/cm) | 0.1 | 1.0 | 5.0 |
| pH at 25°C | – | – | 5.0–7.0 |
| Oxidizable Matter Content (mg/l) | – | 0.08 | 0.4 |
| Absorbance at 254 nm | 0.001 | 0.01 | – |
| Residue After Evaporation (mg/kg) | – | 1 | 2 |
| Silica Content (mg/L) | 0.01 | 0.02 | – |
SatiCus is the exclusive distributor of Stakpure in Vietnam, providing laboratory water purification solutions that meet ISO 3696 and ASTM D1193-06 standards. Utilizing advanced German technology, Stakpure offers systems that deliver consistent, high-precision, and safe water sources for all laboratory requirements, from Type III to ultrapure Type I water.