Dissolution tester
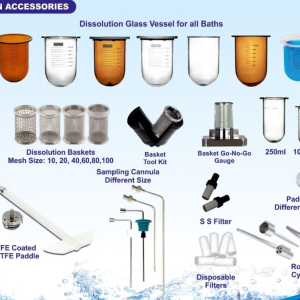
Mahir company specializes in supplying accessories for dissolution equipment.
Dissolution tester
BATHLESS DISINTEGRATION INSTRUMENT DISTEK SENSIR 3200 -DISTEK
The Distek sensIR 3200 combines two exclusive technologies – bathless heating and Near IR sensing to offer the most innovative disintegration tester. Bathless heating not only reduces warm-up times but eliminates the inconveniences associated with conventional water bath-based instruments. To match your throughput requirements, the sensIR 3200 is available in two, four or six positions in the smallest footprint of any comparably equipped model.
Dissolution tester
Highly portable, impaction based microbial air sampler 28.3LPM, 100LPM and 200LPM flow rate pre-selectable Built-in, high efficiency exhaust air filter Complies with ISO 14981-1 and meets EU GMP Annex 1
Dissolution tester
DISSOLUTION MEDIA HEATING, DEGASSING, AND DISPENSING EZFILL+ – DISTEK
Many of the main causes of OOS dissolution tests and qualification failures are related to media deaeration and dispensing. The ezfill+, with method controlled automated deaeration and dispensing, makes media preparation simple and error free. Reports that include method parameters and user information allow tracking your media preparation as part of a complete data integrity solution. All made possible on a stand-alone basis by a built-in controller using a brilliant touch screen. The unique compact design and optional mobile cart lets one easily move the unit from bath to bath, while the available remote dispensing nozzle allows directly filling vessels in place. The ezfill+ is compatible with most dissolution baths on the market.
Dissolution tester
The Distek Model 2500 RTD Dissolution System offers great flexibility and configurability. It can be configured as USP Apparatus 1, 2, 5, and 6, plus intrinsic dissolution. The extensive user, method, and reporting storage capabilities are enabled by a brilliant touchscreen. Patented wireless temperature sensors continuously monitor and display the in-vessel temperature for each vessel. Unparalleled control of the instrument plus automatic storage of component serial numbers and automatic qualification reminders make the Model 2500 RTD robust and reliable enough for QC applications yet flexible enough for R&D use.
Dissolution tester
The Distek Model 2500 Select Bathless Dissolution System is the culmination of three generations of patented bathless technology with extremely short media heating times and dramatically smaller energy use. The system offers great flexibility and configurability. It can be configured as USP Apparatus 1, 2, 5, and 6 plus intrinsic dissolution. Patented wireless temperature sensors continuously monitor and display the in-vessel temperature for each vessel. Unparalleled control of the instrument plus automatic storage of component serial numbers and automatic qualification reminders make the Model 2500 Select robust and reliable enough for QC applications yet flexible enough for R&D use.
Dissolution tester
The Distek Eclipse 5300 Automated Dissolution Sampler provides fast and accurate sampling via precision syringe pumps. By removing valves and rotary pumps and optimizing the sample pathway with a minimal 4.5 mL internal volume, reliability is enhanced and sample carryover is minimized. For economical sampling from two dissolution baths, a more affordable ‘child’ sampler can be added, allowing both baths to run identical or separate methods. Additionally, the optional integrated filter changer replaces tedious manual filtering, and saves space compared to standalone automated filtering units.
Dissolution tester
The DS 14000 (Basic) SMART dissolution tester is the perfect solution for pharmaceutical laboratories, designed to assess tablet dissolution in compliance with international standards such as USP, BP, IP, and JP. Featuring a 12+2 vessel configuration and an automatic tablet dispenser, the device enables comparative studies while ensuring precise and efficient testing processes. It is an ideal choice for quality control and product development in the pharmaceutical industry.